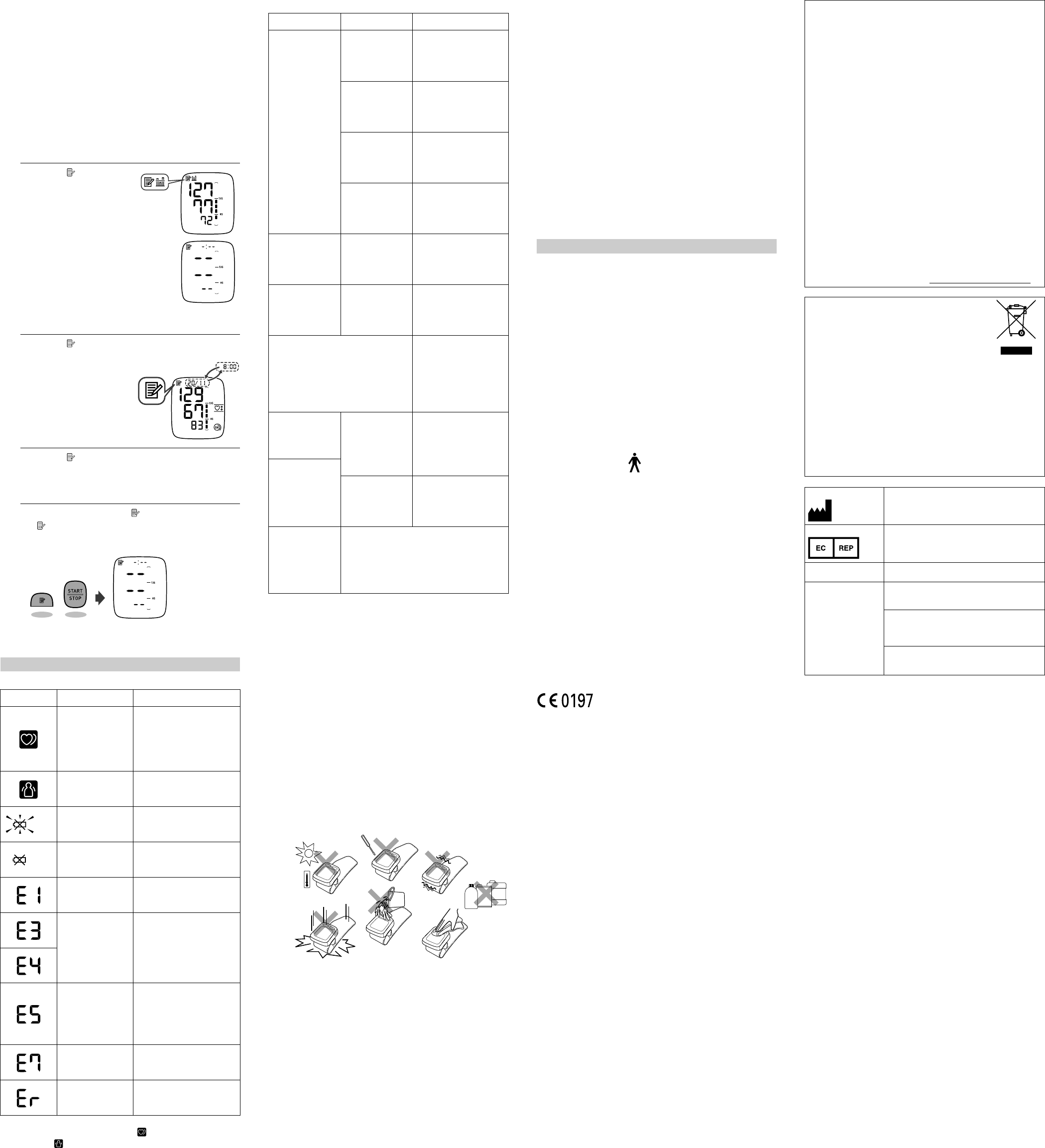
3.5 Using the Memory Function
The monitor automatically s
It can also calculate an
measurements from t
minutes. If th
period, the average
one reading in memory for that period, the average wi
based on one r
Notes:
• If the memory is full, the monitor will delete the oldes
readings.
• When viewing the reading t
time, “-:--” is displayed instead of the date and t
T
T
T
Note: Y
4.1 The Icons and Error Messages
Note: The irregular heartbeat sy ) and
symbol ( ) may also be displayed with error messages.
4.2 T
4.3 Maintenance
T
follo
• Do not sub
extreme
dire
• Do not d
• Do not sub
vibration
floor).
• Do not u
• Do not w
• Do not u
clean th
• Do no
defect occ
or distribu
• The uni
• Use a so
clean th
• Keep the u
use.
• Fold th
Do not st
• If the uni
• Locatio
humidi
vapour
• Locatio
where i
Calibratio
• The a
been car
service
• It is gen
inspected
function
authorise
Customer
packaging
Note: Subject to technical modification without prior notice.
• This device fulfils the provis
(Medical Device Directive).
• This blood pressure monitor is designed
S
General Requirements and P
for electromechanical
• T
OMRON HEAL
OMRON blood pressich is the Pressure Sensor
produced in Japan.
Made in China
Press the
Note: If there are no
results stored in the
screen to t
1.
Press bu
display
The Memory number
appears for a second
before the pulse rate is
displayed. The newest
set is numbered “1”.
2.
Press bu
readings s
When th )
the
button.
ST
second
Error Display Cause Remedy
Irregular heartbeats
are detected.
Remove the wrist cuf
2 - 3 minutes and t
another measurem
Repeat the steps in section
3.3. If this error c
appear
Movemen
measurement.
Carefully read and repeat the
steps in section 3.3.
Blink
The batteries are
low
Y
new ones ahead of time.
Refer to sect
Lit
The batteries are
exhausted.
Y
new ones at once.
Refer to sect
Wrist
applied correctly
Apply the wrist cuff correctly
Refer to sect
Movemen
measurement.
Repeat Measurement
remaining still and refraining
from talking during
measurement.
Refer to sect
Wrist
applied correctly
movemen
measurement.
Apply the wrist cuff correctly
and repeat mea
while remaining still and
refraining from taking during
the measurement.
Refer to sect
Arm position
changed during
measurement.
Remain still until
measurement is complete.
Refer to sect
Device error
Contact your OMRON
outlet or distributor
Alternating
date/time
display
4. T
Problem Cause Remedy
The reading is
extremely low
(or high).
The wrist cuff is
at heart level.
Measure while in the
corr
Refer to section 3.2.
The cuff is not
wrapped sn
around the wris
Wrap the cuff correctly
Refer to section 3.1.
The arms
shoulders a
tense.
Relax and try taking the
measurement again.
Refer to section 3.3.
Move
talking during
measurement.
Remain still and do not
talk during Measurement.
Refer to section 3.3.
Wrist c
does not rise.
Air is leaking f
the wrist cuff.
Consult your OMRON
retail outlet or dist
Wrist c
too soon.
The
loose.
Apply the cuff correctly so
that it is
around the wrist. Refer to
section 3.1.
The blood pressu
each time. The reading is
extremely low (or high)
Blood pressur
constantly vary with ti
of day and how relaxed
you are. T
deep brea
remain relaxed before
taking a measurement.
The unit loses
power during
measurement.
The batteries a
exhausted.
Replace the batteries with
new ones.
Refer to section 2.1.
Nothing happens
when you press
the buttons.
The batteries have
been inserted
incorrectly
Insert the batteries with
the correct (+/-) polarity
Refer to section 2.1.
Other problems.
• Press the ST
measurement.
• If the problem
batteries with new ones.
If this still does not solve the problem, cont
your OMRO
Product Descript Wrist Blood Pressur
Model OMRON RS
Display LCD Digit
Measurement M Oscillometric me
Measurement Range Pressure:
Pulse: 40 to 180 beat
Accuracy Pressure: ±
Pulse: ±5% of dis
Inflation Automatic inflation by pump
Deflation Automatic rapid deflation
Memory 90 Measurements
Power Source 2 x 1.5V (LR03, AAA alkaline batteries)
Battery Life Approx. 300
alkaline batteries at a room temperature of
23°C
Applied Part = T
Protection Against
Ele
Internally powered ME equipment
Operating tempe
Humidity
+10 to +4
S
Humidity/Air pressure
-20
700 to 1060hPa
Console Weight Approximately 85 g without batteries
Outer D Approximately
14 (d) mm
Measurable
circ
Approximately 13.5 to 21.5 cm
Cuff Material Nylon and polyester
Package Cont M
instruction manual, guarant
pressure pass
Important information regarding Electro Magnetic
Comp
With the increased number of electronic devices suc
mobile (cellular) telephones, medical devices in use may be
susceptible to electromagnetic interference f
Electromagnetic interference
the medical device and
Medical devices
In order to
Compatibility) with the aim to prevent unsaf
EN60601-1-2:2007 standard has b
defines the levels of immunity to electromagnetic interferences as
well as maximum
devices.
This medical device manufac
conforms to this EN60601-1-
emissions.
Nevertheless, special precautions need to be observed:
• Do not telephones and other devices, which
generate strong electrical orelectromagnetic fields, near the
medical device. T
and create a potentially uns
keep a minimum distance of 7
device in case t
Further documentation in ac
available at OMRON HEAL
mentioned in this instruc
Documentation is also ava
Correct
(Waste Electrical & Electro
This marking shown on the product or its
literature, indicates that it s
disposed of, with ot
end of its working life.
T
uncontrolled waste dispos
other types of was
sustainable reuse of material resources.
Household users
purchased this product, or their local government office, for
of where and
recycling.
Business users should contact their supplier and check the terms
and conditions of the purchase cont
mixed with other commercial wastes for disposal.
Manufacturer
OMRON HEAL
53, Kunotsubo, T
617-
EU-representative
OMRON HEAL
Sc
THE NETHERLANDS
www
Product
OMRON (DALIAN) CO.,
Dalian, CHINA
Subsid
OMRON HEAL
Op
U.K.
OMRON MEDIZINTECHNIK
HANDELSGESELLSCHAFT mbH
John-De
www
OMRON SANTÉ FRANCE SAS
14, rue de
Cedex, FRANCE
HEM-6221-E.book Page 2 Tuesday, November 6, 2012 2:06 PM